Puregraft announced today results from the MICROPREP Clinical Trial: Intra-articular Injection of Fat and Platelet Rich Plasma as a Treatment for Knee Osteoarthritis. This phase IIa study was conducted by Dr Marie Laure Louis, orthopedic surgeon in Marseille, France (Clinique Juge, Almaviva group). The MICROPREP results will be presented by Dr. Jeremy Magalon (Assistance Publique – Hopitaux de Marseille) at the upcoming International Orthopedics Foundation Annual Meeting in Denver, Colorado.
The MICROPREP study was a 30 patient, prospective, randomized, double-blind, single-center clinical trial evaluating three treatment groups for patients diagnosed with osteoarthritis: Puregraft processed fat + saline, Puregraft processed fat + 1Billion Platelets, and Puregraft processed fat + 3Billion platelets were injected into the knee under local anesthesia. Preliminary results of the study were recently presented at the annual IFATS meeting in Marseille, France (December 2019). A reduction in pain and improvement in function, as measured by PAIN VAS and WOMAC scales, along with MRI results were observed among all three treatment groups at six months. No serious adverse events were reported and no significant difference between the three types of treatments were observed. According to Dr. Magalon, “All three treatment arms showed improvement.”
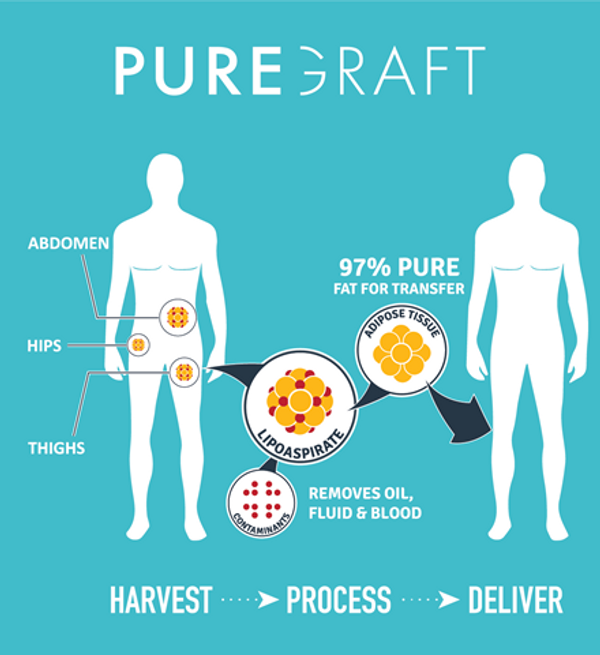
“Puregraft processed microfat appears to be a viable treatment for patients suffering from osteoarthritis of the knee” noted Brad Conlan, CEO of Puregraft. “Patients with osteoarthritis lose the protective cushion in their knee joint, resulting in debilitating grinding of bone on bone. This clinical data reinforces what we already know–Puregraft processed fat is a therapeutic volumizer, aiming to restore normal movement and quality of life.”
The Joint Pain Injection Market
The company is pursuing the further clinical and market development of Puregraft in the osteoarthritis marketplace, where nearly 30 million adults suffer in the United States alone. Osteoarthritis (OA) is the most common form of arthritis. It occurs most frequently in the hands, hips, and knees. With OA, the cartilage within a joint begins to break down and the underlying bone begins to change. These changes usually develop slowly and get worse over time. OA can cause pain, stiffness, and swelling. In some cases, it also causes reduced function and disability; some people are no longer able to do daily tasks or work.1 The global joint pain injections market is currently valued at over $2.1B and is expected to grow to $5.7B.2
About Puregraft
Puregraft is a private company owned by Bimini Health Tech. In 15 minutes or less, the company's FDA cleared, and CE-Marked technology can provide a surgeon with purified adipose tissue for reinjection. Puregraft technology is used in hospitals and clinics around the world. Puregraft is positioned to continue its leadership in the rapidly expanding, fat grafting marketplace.
Learn more about Puregraft at http://www.puregraft.com
1. The US Center for Disease Control and Prevention. 2. Transparency Market Research (TMR) has published a new report titled, “Joint Pain Injections Market – Global Industry Analysis, Size, Share, Growth, Trends, and Forecast, 2018–2026